The Medicines Authority in Aden withdraws four Indian medical preparations from the market due to its violation of the specifications
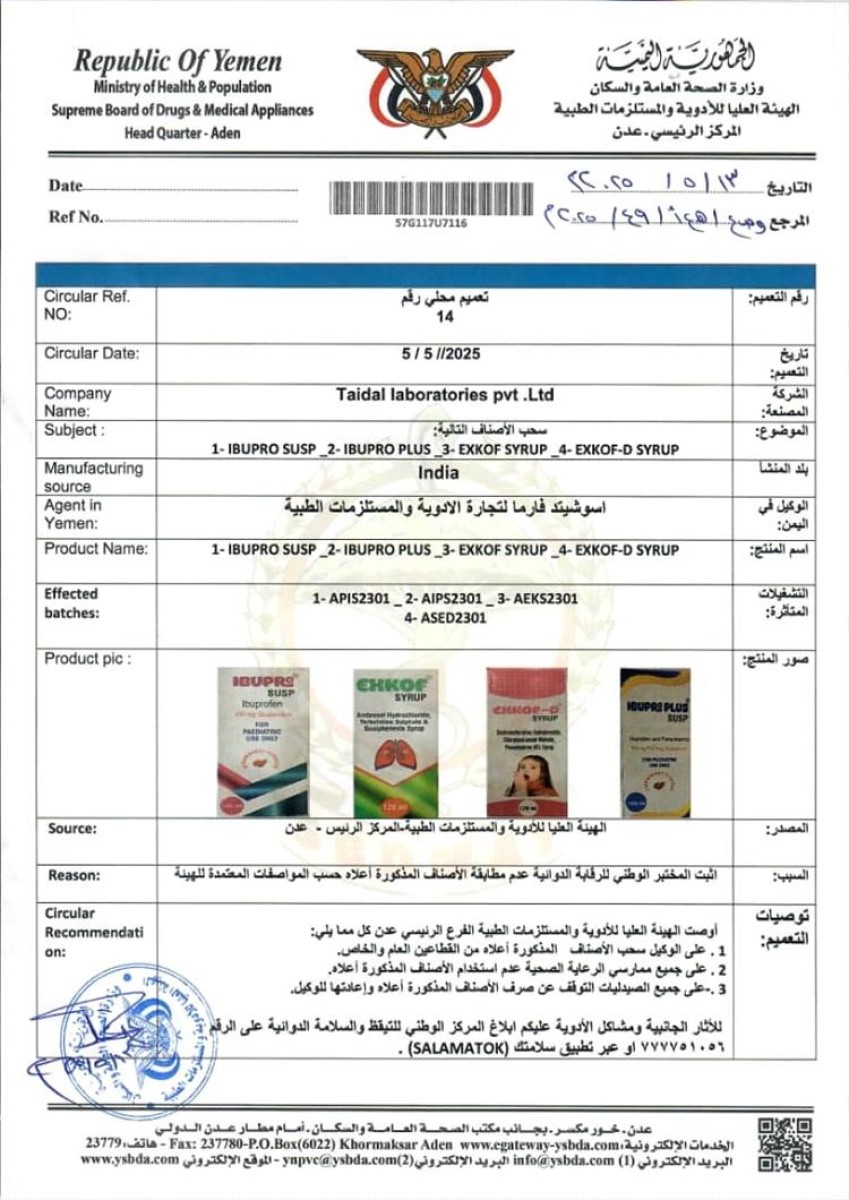

The High Authority for Medicines and Medical Supplies in the capital, Aden, issued a circular by withdrawing four medical preparations produced by the Indian company Taidal Laboratories, after it was proven that it did not conform to the approved specifications.
It came in the circular, which carried the number (14) issued on May 5, 2025, that the products that are decided to withdraw from the market are:
1. Ibupro Susp
2. Ibupro plus
3. EXKOF Syrup
4. EXKOF-D Syrup
The authority indicated that the reason for the withdrawal is due to reports from the National Center for Drug Control, confirming that the items mentioned do not match the specifications approved by the authority.
The authority stressed the agents and importers of medicines and medical supplies not to sell, use or distribute these items, with the need to inform the competent authorities of any quantities available from them with pharmacies and health facilities. It also called for informing the National Center for Pharmaceutical Safety and Safety of any side effects resulting from the use of these preparations via the number (777751056).
The authority affirmed its commitment to follow up the quality and safety of medicines in order to preserve the health and safety of citizens, calling on pharmacists and citizens to cooperate and report any violations.